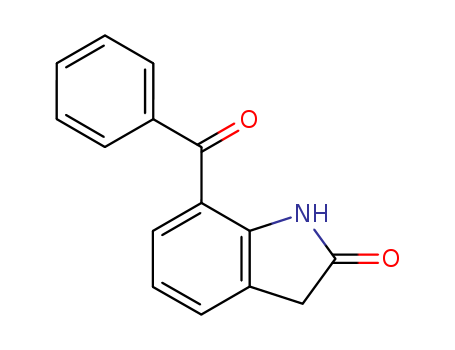
51135-38-7
- Product Name:7-benzoyl-2-oxindole
- Molecular Formula:C15H11NO2
- Purity:99%
- Molecular Weight:237.258
Product Details;
CasNo: 51135-38-7
Molecular Formula: C15H11NO2
Factory Sells Best Quality 7-benzoyl-2-oxindole 51135-38-7 with stock
- Molecular Formula:C15H11NO2
- Molecular Weight:237.258
- Vapor Pressure:3.39E-10mmHg at 25°C
- Melting Point:154℃
- Refractive Index:1.627
- Boiling Point:501.7 °C at 760 mmHg
- PKA:13.42±0.20(Predicted)
- Flash Point:214.3 °C
- PSA:46.17000
- Density:1.254 g/cm3
- LogP:2.55020
7-BENZOYL-1,3-DIHYDRO-INDOL-2-ONE(Cas 51135-38-7) Usage
InChI:InChI=1/C15H11NO2/c17-13-9-11-7-4-8-12(14(11)16-13)15(18)10-5-2-1-3-6-10/h1-8H,9H2,(H,16,17)
51135-38-7 Relevant articles
Conformational and crystal energetics of a polymorphic cyclized product of Napafenac: The Z′ and crystal stability correlation
Nanubolu, Jagadeesh Babu,Ravikumar, Krishnan,Sridhar, Balasubramanian,Sreedhar, Bojja
, p. 133 - 145 (2014)
We report the single crystal diffraction...
Preparation method of 7-benzoyl-1,3-indoline-2-ketone
-
Paragraph 0031; 0032, (2016/11/14)
The invention discloses a preparation me...
Palladium-catalyzed direct addition of arylboronic acids to 2-aminobenzonitrile derivatives: Synthesis, biological evaluation and in silico analysis of 2-aminobenzophenones, 7-benzoyl-2-oxoindolines, and 7-benzoylindoles
Chen, Jiuxi,Ye, Leping,Su, Weike
supporting information, p. 8204 - 8211 (2015/01/08)
A palladium-catalyzed direct addition of...
Antiinflammatory agents. 3. Synthesis and pharmacological evaluation of 2-amino-3-benzoylphenylacetic acid and analogues
Walsh,Moran,Shamblee,Uwaydah,Welstead Jr.,Sancilio,Dannenburg
, p. 1379 - 1388 (2007/10/02)
A series of substituted derivatives of 2...
51135-38-7 Process route
-
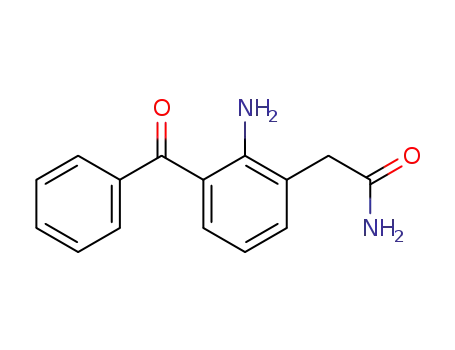
-
78281-72-8
nepafenac

-
-
51135-38-7
7-benzoyl-1,3-dihydroindol-2-one
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
ethanol; water;
at 30 ℃;
for 3h;
Temperature;
Reagent/catalyst;
Solvent;
|
92.1% |
|
With
hydrogenchloride;
In
ethanol;
Heating;
|
-
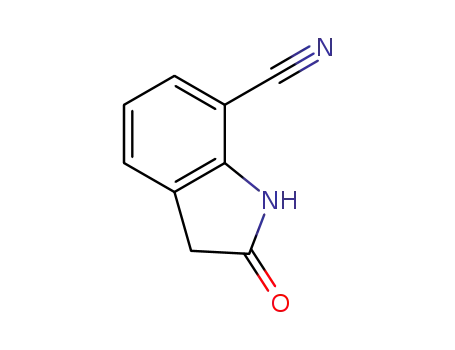
-
380427-40-7
2-oxoindoline-7-carbonitrile

-
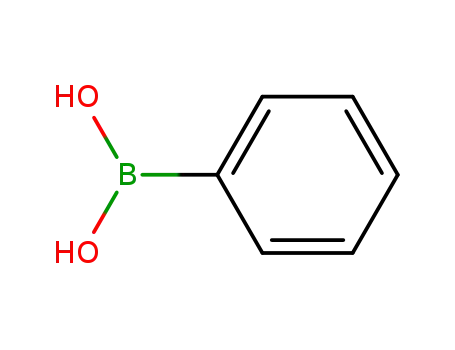
-
98-80-6
phenylboronic acid

-

-
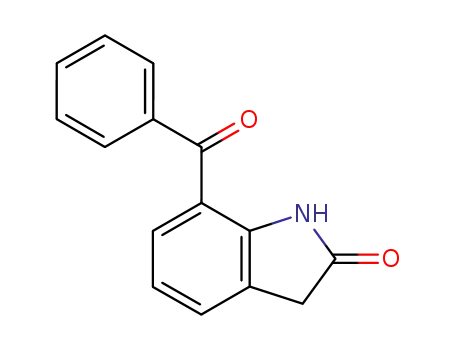
51135-38-7
7-benzoyl-1,3-dihydroindol-2-one
| Conditions | Yield |
|---|---|
|
With
5,5'-dimethyl-2,2'-bipyridine; methanesulfonic acid; palladium(II) trifluoroacetate; water;
In
2-methyltetrahydrofuran;
at 80 ℃;
for 36h;
Schlenk technique;
|
89% |
51135-38-7 Upstream products
-
50-00-0
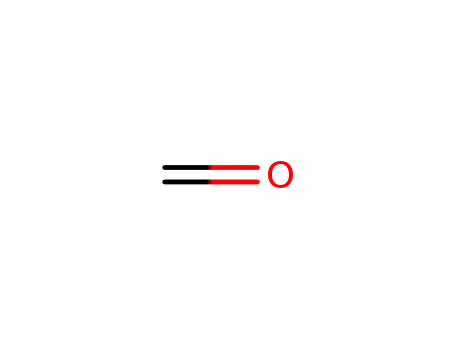
formaldehyd
-
61941-57-9
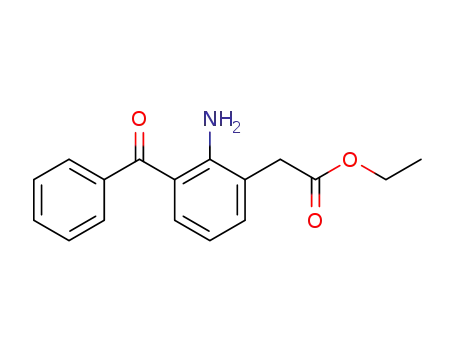
2-amino-3-benzoylbenzeneacetic acid,ethyl ester
-
61085-33-4
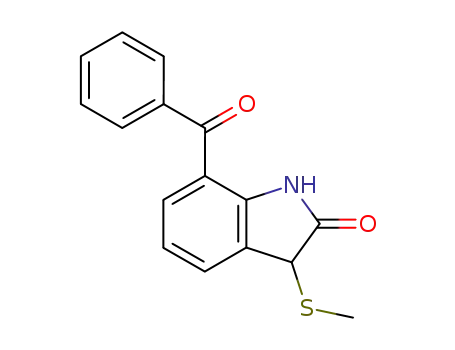
7-benzoyl-3-methylsulfanyl-1,3-dihydro-indol-2-one
-
76049-81-5
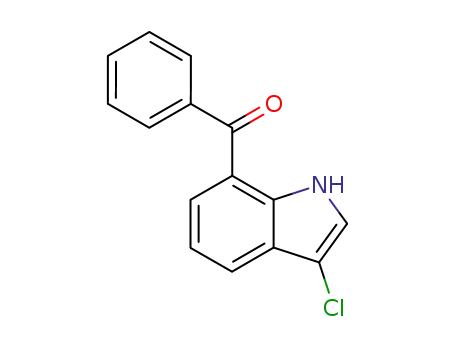
3-chloro-7-benzoylindole
51135-38-7 Downstream products
-
51579-82-9
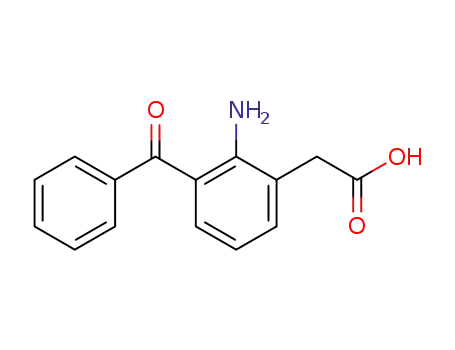
amfenac
-
104039-30-7
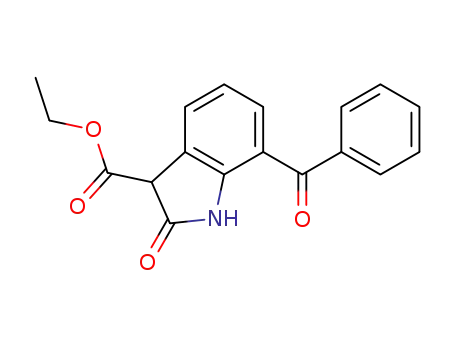
Ethyl 7-benzoyloxindole-3-carboxylate
-
61941-56-8
2-amino-3-benzoylbenzeneacetic acid,sodium salt
Relevant Products
-
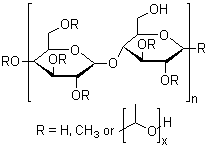
Sodium carboxymethyl cellulose,CMC-Na
CAS:9004-32-4
-
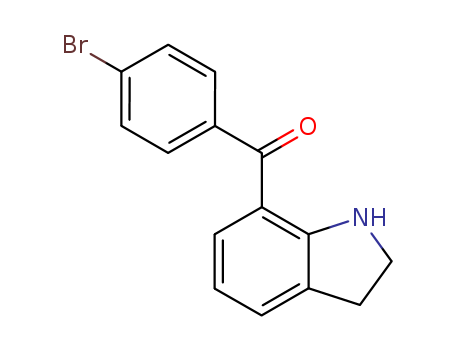
(4-bromophenyl)(indolin-7-yl)methanone
CAS:91714-41-9
-
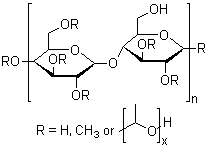
HEC 10M
CAS:9004-62-0