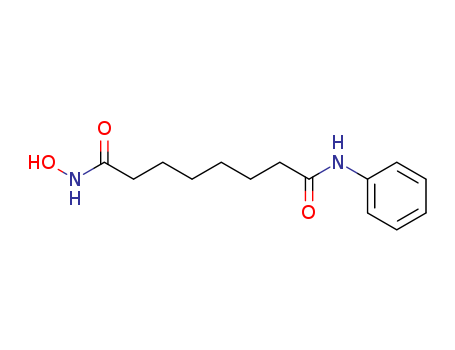
149647-78-9
- Product Name:Vorinostat
- Molecular Formula:C14H20N2O3
- Purity:99%
- Molecular Weight:264.324
Product Details;
CasNo: 149647-78-9
Molecular Formula: C14H20N2O3
Appearance: white crystalline solid
Manufacturer supply top purity Vorinostat 149647-78-9 with GMP standards
- Molecular Formula:C14H20N2O3
- Molecular Weight:264.324
- Appearance/Colour:white crystalline solid
- Melting Point:161-162 °C
- Refractive Index:1.566
- PKA:9.48±0.20(Predicted)
- PSA:78.43000
- Density:1.174 g/cm3
- LogP:2.93500
Vorinostat(Cas 149647-78-9) Usage
|
Antitumor drugs |
Vorinostat is a novel, molecularly targeted antineoplastic agent that causes cell cycle arrest and/or apoptosis by inhibiting histone deacetylase (HDAC). It is the first HDAC inhibitor approved by the US Food and Drug Administration (FDA) for the treatment of cutaneous T-cell lymphoma (CTCL) with significant skin involvement that is still progressing, resistant or relapsing after two systemic regimens. On October 6, 2006, the US Food and Drug Administration (FDA) have approved vorinostat capsules (vorinostat) for the treatment of skin cancer drugs. The drug is the first novel type of anti-cancer drugs of histone deacetylase inhibitor developed by the United States Merck for the treatment of skin T cell lymphoma (CTCL). FDA has approved it for the treatment of metastatic skin T-cell lymphoma which is unable to be cured or even worsened or gets recurrent cases. A large number of experimental studies and clinical results have shown that vorinostat has a excellent efficacy on a variety of tumors and have significant synergies when combined with other oncology drugs. The current treatment of other tumors is still undergoing in-depth study; these results show that vorinostat has a broad market prospects. Vorinostat has low toxicity with the evidence of its safety and efficacy being supported by two clinical trials, including 107 patients with CTCL who had gotten relapsed after receiving other drugs. According to the standard analysis of improvement in the grade of skin lesion, 30% of patients treated with Zolinza get symptoms improved, with the average efficacy duration of 168 days. The most common serious adverse events were pulmonary embolism, dehydration, deep venous thrombosis and anemia. Common adverse reactions are gastrointestinal symptoms (including diarrhea, nausea and loss of appetite, vomiting and constipation); fatigue, chills and taste disorders. Animal experiments showed that pregnant women should be banned of using the drug. |
|
Preparation |
Suberic acid can undergo the intramolecular dehydration into suberic anhydride under the action of the acetic anhydride. The suberic anhydride, together with aniline can have ring-opening amidation in ethyl acetate at 0 °C to generate suberic acid monoanilide, followed by methanol esterification and hydroxylamine amine aminolysis to obtain the anti-tumor drug in vorinostat with the total yield of about 65%. "Chinese Journal of Pharmaceutical Industry" 2009, Volume 40, No. 7, pages 481-483 |
|
Anti-cancer drug Vorinostat was able to clear latent HIV virus |
Researchers from the University of North Carolina at Chapel Hill have published a groundbreaking research paper in the July 25, 2012 issue of Nature to confirm that a deacetylase inhibitor drug – vorinostat that can be used to treat certain types of lymphoma-being capable of clearing out the patient's latent HIV virus in vivo. The researchers have conducted a series of experiments to evaluate the potential of this drug to activate and destroy latent HIV viruses. Initially, laboratory experiments for measuring the level of active HIV in CD4 + T cells showed that vorinostat can take off the camouflage of latent HIV viruses in these cells. Then, eight male patients who still kept medically stable HIV infection after antiretroviral therapy, took vorinostat, and then were tested their active HIV levels in the body and compared it to the levels they had before taking the drug. The researchers found that HIV-RNA levels in CD4 + T cells increased by an average of 4.5-fold in those patients who receiving vorinostat, confirming that the HIV virus was disguised. This is the first published study confirming that deacetylase inhibitors have the potential to break down latency in latent virus libraries. The study provides convincing evidence that a new strategy may be used to directly attack and eradicate latent HIV infection. However, getting rid of the latent nature of HIV is only the first step in curing HIV infection. |
|
Biochem/physiol Actions |
SAHA or Vorinostat facilitates the transcription of genes that result in apoptosis, differentiation and growth arrest. It has been observed to give beneficial results in lymphoma but not in solid tumors. |
|
Synthesis |
Commercially available monomethyl ester 125 was reacted with aniline in the presence of DCC and HOBt in DMF to give amide 127 in 89% yield.Methyl ester amide 127 was then reacted with hydroxylamine HCl salt and potassium hydroxide in methanol to give vorinostat (XVI) in 90% yield. |
|
Definition |
ChEBI: A dicarboxylic acid diamide comprising suberic (octanedioic) acid coupled to aniline and hydroxylamine. A histone deacetylase inhibitor, it is marketed under the name Zolinza for the treatment of cutaneous T cell lymphoma (CTCL). |
|
Brand name |
Zolinza |
|
General Description |
Histones are proteins around which DNA is wound in the process of packing DNA into the nucleus. They also havea role in regulating the transcription of genes, and this iscontrolled by the covalent modifications acetylation, phosphorylation,and methylation to which they are subject.Vorinostat fits the basic pharmacophore for the HDACis, which consists of a hydrophobic cap regionconnected to a zinc coordinating functionality by a hydrophobiclinker.The hydroxamic acid functionality iscapable of bidendate binding to zinc present in the enzymeand is a major factor in the overall binding of the compound.The compound inhibits HDAC1, 2, 3, and 6 classes of thisenzyme with nanomolar (<86 nM) IC50 values.The agent is given orally and is available in 100-mg capsulesfor the treatment of cutaneous T-cell lymphoma. Thebioavailability is 43%, and the agent is 71% bound toplasma proteins. Extensive metabolism of the agent occursto give the O-glucuronide of the hydroxamic acid functionand 4-anilino-4-oxobutanoic acid with minimal involvementof isozymes of CYP. The metabolites, both of whichare inactive, are eliminated in the urine and the drug has aterminal elimination half-life of 2 hours. The most commonlyreported adverse effects are fatigue, diarrhea, andnausea. |
InChI:InChI=1/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18)
149647-78-9 Relevant articles
Novel SAHA analogues inhibit HDACs, induce apoptosis and modulate the expression of microRNAs in hepatocellular carcinoma
Srinivas, Chatla,Swathi,Priyanka,Anjana Devi,Subba Reddy,Janaki Ramaiah,Bhadra, Utpal,Bhadra, Manika Pal
, p. 1249 - 1264 (2016)
In eukaryotes, transcriptional regulatio...
α,?-Didehydrosuberoylanilide hydroxamic acid (DDSAHA) as precursor and possible analogue of the anticancer drug SAHA
Chattopadhyay, Shital K.,Ghosh, Subhankar,Sarkar, Sarita,Bhadra, Kakali
, p. 2524 - 2533 (2019)
An alternate synthetic route to the impo...
A nanodelivered Vorinostat derivative is a promising oral compound for the treatment of visceral leishmaniasis
Corpas-López, Victoriano,Díaz-Gavilán, Mónica,Franco-Montalbán, Francisco,Merino-Espinosa, Gemma,López-Viota, Margarita,López-Viota, Julián,Belmonte-Reche, Efres,Pérez-del Palacio, José,de Pedro, Nuria,Gómez-Vidal, José Antonio,Morillas-Márquez, Francisco,Martín-Sánchez, Joaquina
, p. 375 - 383 (2019)
There is currently no satisfactory treat...
Efficient continuous flow synthesis of hydroxamic acids and suberoylanilide hydroxamic acid preparation
Riva, Elena,Gagliardi, Stefania,Mazzoni, Caterina,Passarella, Daniele,Rencurosi, Anna,Vigo, Daniele,Martinelli, Marisa
, p. 3540 - 3543 (2009)
A continuous flow tubing reactor can be ...
An Endogenous Reactive Oxygen Species (ROS)-Activated Histone Deacetylase Inhibitor Prodrug for Cancer Chemotherapy
Bhagat, Somnath D.,Singh, Usha,Mishra, Ram Kumar,Srivastava, Aasheesh
, p. 2073 - 2079 (2018)
Suberoylanilide hydroxamic acid (SAHA, v...
A new simple and high-yield synthesis of suberoylanilide hydroxamic acid and its inhibitory effect alone or in combination with retinoids on proliferation of human prostate cancer cells
Gediya, Lalji K.,Chopra, Pankaj,Purushottamachar, Puranik,Maheshwari, Neha,Njar, Vincent C. O.
, p. 5047 - 5051 (2005)
We have developed a procedure for the sy...
Hypoxia-activated pro-drugs of the KDAC inhibitor vorinostat (SAHA)
Calder, Ewen D. D.,Conway, Stuart J.,Folkes, Lisa K.,Hammond, Ester M.,Mistry, Ishna N.,Skwarska, Anna,Sneddon, Deborah
supporting information, (2020/04/24)
Hypoxia (lower than normal oxygen) is a ...
Histone Deacetylase 2 (HDAC2) Inhibitors Containing Boron
Kavianpour, Poya,Gemmell, Madeleine C. M.,Kahlert, Jan U.,Rendina, Louis M.
, p. 2786 - 2791 (2020/06/25)
Histone deacetylase enzymes (HDACs) are ...
149647-78-9 Process route
-
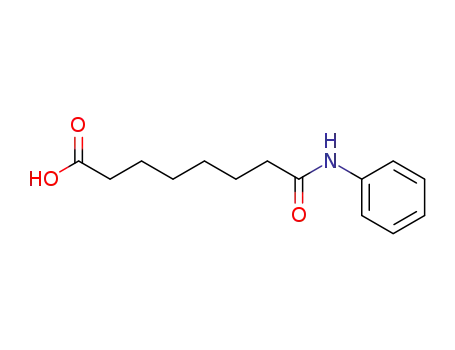
-
149648-52-2
suberanilic acid

-
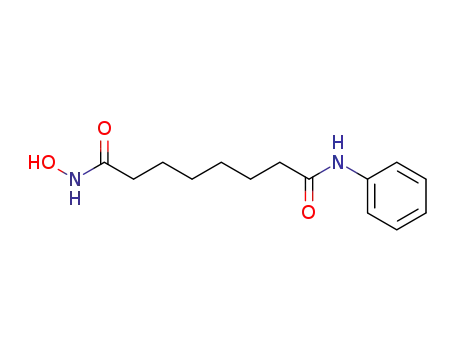
-
149647-78-9
vorinostat
| Conditions | Yield |
|---|---|
|
With
C36H24B4N2O3; hydroxylamine hydrochloride; triethylamine;
In
toluene;
at 80 ℃;
for 14h;
Molecular sieve;
|
82% |
|
suberanilic acid;
With
1,1'-carbonyldiimidazole;
In
N,N-dimethyl-formamide;
at 25 - 30 ℃;
for 0.5h;
With
hydroxylamine hydrochloride;
In
N,N-dimethyl-formamide;
for 0.5h;
|
75% |
|
suberanilic acid;
With
1,1'-carbonyldiimidazole;
In
N,N-dimethyl-formamide;
at 25 - 30 ℃;
for 0.5h;
With
hydroxylamine hydrochloride;
In
N,N-dimethyl-formamide;
for 0.5h;
|
75% |
|
suberanilic acid;
With
dmap; ethyl 2-(tert-butoxycarbonyloxyimino)-2-cyanoacetate; N-ethyl-N,N-diisopropylamine;
In
tetrahydrofuran;
at 0 - 5 ℃;
for 0.5h;
With
hydroxylamine hydrochloride;
In
tetrahydrofuran; N,N-dimethyl-formamide;
at 20 ℃;
|
68% |
|
suberanilic acid;
With
chloroformic acid ethyl ester; triethylamine;
In
tetrahydrofuran;
at 0 ℃;
for 0.5h;
With
hydroxylamine;
In
methanol;
at 20 ℃;
for 1h;
|
62% |
|
Multi-step reaction with 2 steps
1: Et3N / tetrahydrofuran / 0.17 h
2: NH2OH / methanol
With
hydroxylamine; triethylamine;
In
tetrahydrofuran; methanol;
|
|
|
Multi-step reaction with 5 steps
1: triethylamine / tetrahydrofuran / 0.25 h / -10 °C
2: sodium tetrahydroborate / methanol / 0.5 h / 0 °C
3: Dess-Martin periodane / dichloromethane / 1 h / 20 °C
4: Benzoylformic acid / Petroleum ether / 48 h / 20 °C / Irradiation; Green chemistry
5: hydroxylamine hydrochloride; triethylamine / dichloromethane / 24 h / 20 °C
With
sodium tetrahydroborate; hydroxylamine hydrochloride; Dess-Martin periodane; triethylamine; Benzoylformic acid;
In
tetrahydrofuran; methanol; dichloromethane; Petroleum ether;
|
|
|
suberanilic acid;
With
1,1'-carbonyldiimidazole;
In
N,N-dimethyl-formamide;
at 25 ℃;
for 0.5h;
With
hydroxylamine hydrochloride;
In
N,N-dimethyl-formamide;
for 0.5h;
|
|
|
Multi-step reaction with 2 steps
1.1: O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine / tetrahydrofuran / 0 °C / Cooling with ice
1.2: 20 °C
2.1: dihydrogen peroxide / aq. phosphate buffer / 4 h / 37 °C / pH 7.4
With
dihydrogen peroxide; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine;
In
tetrahydrofuran; aq. phosphate buffer;
|
|
|
Multi-step reaction with 2 steps
1: Dowex50W-X2 acid resin / 22 h / Reflux
2: methanol; hydroxylamine hydrochloride; sodium
With
methanol; hydroxylamine hydrochloride; sodium;
|
|
|
Multi-step reaction with 2 steps
1: 18 h / Heating / reflux
2: hydroxylamine hydrochloride; sodium methylate / methanol
With
hydroxylamine hydrochloride; sodium methylate;
In
methanol;
|
|
|
suberanilic acid;
With
chloroformic acid ethyl ester; triethylamine;
at 0 ℃;
With
hydroxylamine;
In
methanol;
at 20 ℃;
|
-
-
C17H23NO5

-

-
149647-78-9
vorinostat
| Conditions | Yield |
|---|---|
|
With
hydroxylamine;
In
methanol;
|
|
|
With
hydroxylamine hydrochloride; potassium hydroxide;
In
methanol;
at 20 ℃;
for 0.25h;
|
|
|
Multi-step reaction with 4 steps
1: sodium tetrahydroborate / methanol / 0.5 h / 0 °C
2: Dess-Martin periodane / dichloromethane / 1 h / 20 °C
3: Benzoylformic acid / Petroleum ether / 48 h / 20 °C / Irradiation; Green chemistry
4: hydroxylamine hydrochloride; triethylamine / dichloromethane / 24 h / 20 °C
With
sodium tetrahydroborate; hydroxylamine hydrochloride; Dess-Martin periodane; triethylamine; Benzoylformic acid;
In
methanol; dichloromethane; Petroleum ether;
|
|
|
With
hydroxylamine hydrochloride;
In
tetrahydrofuran; methanol;
at 20 ℃;
for 1.5h;
|
|
|
With
hydroxylamine hydrochloride; potassium hydroxide;
In
tetrahydrofuran; methanol;
at 20 ℃;
for 0.5h;
|
0.14 g |
149647-78-9 Upstream products
-
162853-41-0
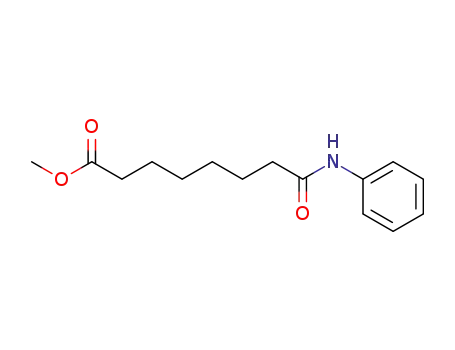
8-oxo-8-(phenylamino)octanoic acid methyl ester
-
3946-32-5
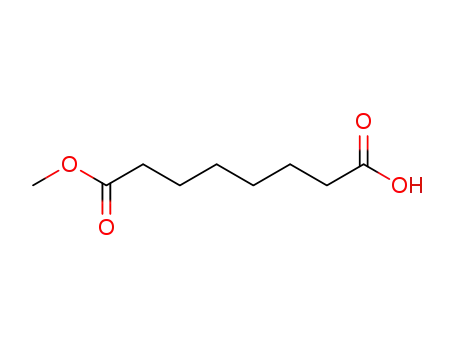
suberic acid monomethyl ester
-
62-53-3
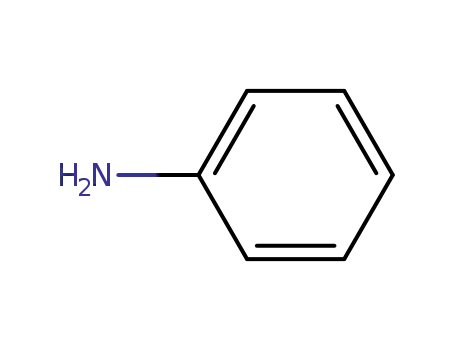
aniline
-
505-48-6
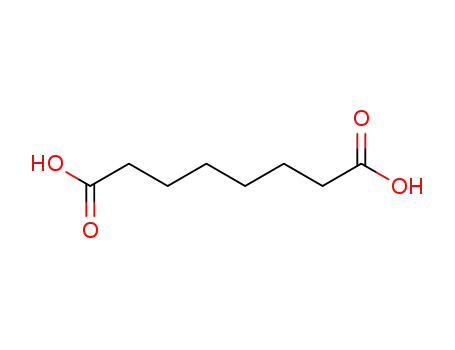
octane-1,8-dioic acid
149647-78-9 Downstream products
-
866824-87-5
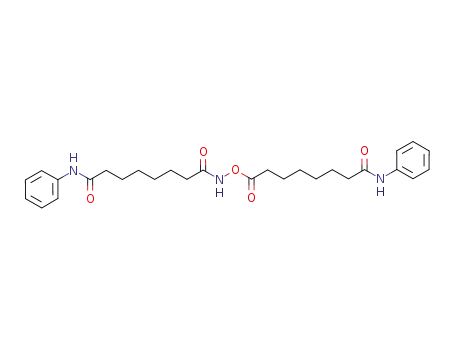
N-[(8-anilino-8-oxooctanoyl)oxy]-N'-phenyloctanediamide
-
1200803-45-7
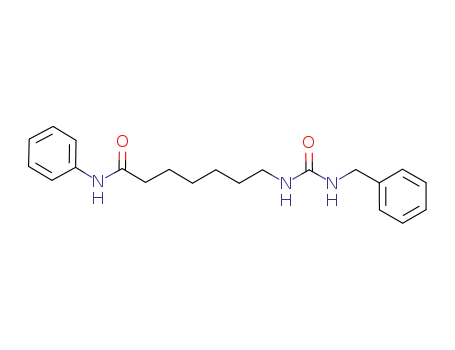
C21H27N3O2
Relevant Products
-
Sodium carboxymethyl cellulose,CMC-Na
CAS:9004-32-4
-
Nifedipine
CAS:21829-25-4
-
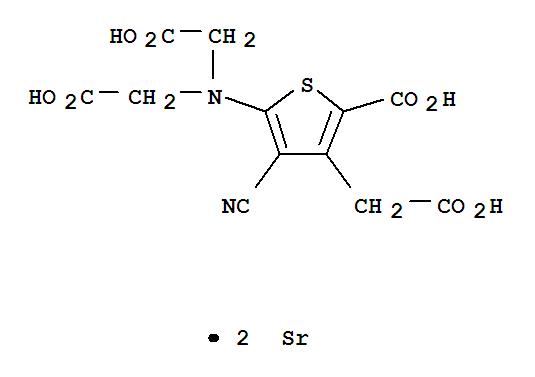
Strontium ranelate
CAS:135459-87-9