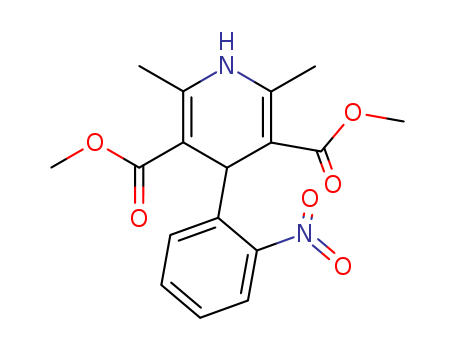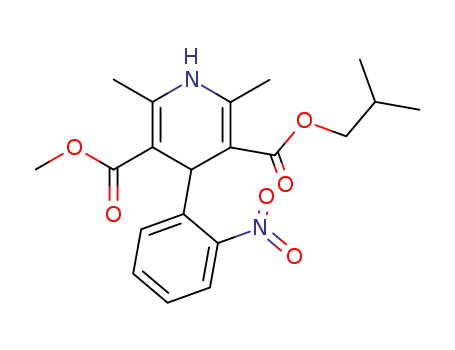
63675-72-9
- Product Name:Nisoldipine
- Molecular Formula:C20H24N2O6
- Purity:99%
- Molecular Weight:388.42
Product Details;
CasNo: 63675-72-9
Molecular Formula: C20H24N2O6
Appearance: light tan crystals
Manufacturer supply high quality Nisoldipine 63675-72-9 with ISO standards
- Molecular Formula:C20H24N2O6
- Molecular Weight:388.42
- Appearance/Colour:light tan crystals
- Vapor Pressure:2.95E-10mmHg at 25°C
- Melting Point:147-148 °C
- Refractive Index:1.544
- Boiling Point:503.3 °C at 760 mmHg
- PKA:2.67±0.70(Predicted)
- Flash Point:258.2 °C
- PSA:110.45000
- Density:1.205 g/cm3
- LogP:4.05380
Nisoldipine(Cas 63675-72-9) Usage
|
Manufacturing Process |
Boiling a solution of 12.7 g of 2'-nitrobenzylideneacetoacetic acid methyl ester and 7.1 g of β-amino-crotonic acid isopropyl ester in 50 ml of methanol for 10 hours yielded 2,6-dimethyl-4-(2'-nitrophenyl)-1,4-dihydropyridine-3,5- dicarboxylic acid 3-methyl ester-5-isopropyl ester of melting point 174°C (from ethanol). Yield 48% of theory. |
|
Therapeutic Function |
Coronary vasodilator |
|
Biochem/physiol Actions |
L-type (CaV1.2) calcium channel blocker; dihydropyridine-type calcium channel blocker with selectivity for smooth muscle (CaV1.2b) over cardiac muscle (CaV1.2a). Arterial vasodilator and antihypertensive agent. |
|
Definition |
ChEBI: A dihydropyridine that is 1,4-dihydropyridine which is substituted by methyl groups at positions 2 and 6, a methoxycarbonyl group at position 3, an o-nitrophenyl group at position 4, and an isobutoxycarbonyl group at position 5. The racemate, calcium channel blocker, is used in the treatment of hypertension and angina pectoris. |
|
Brand name |
Sular (Sciele);Baymycard. |
|
General Description |
In vitro studies show that the effects ofnisoldipine, 1,4-dihydro-2, 6-dimethyl-4-(2-nitrophenyl)-3,5-pyridinecarboxylic acid methyl 2-methylpropyl ester(Sular), on contractile processes are selective, with greaterpotency on vascular smooth muscle than on cardiac muscle.Nisoldipine is highly metabolized, with five majormetabolites identified. As with most of the dihydropyridines,the cytochrome P450 (CYP) 3A4 isozyme is mainlyresponsible for the metabolism of nisoldipine. The majorbiotransformation pathway appears to involve the hydroxylationof the isobutyl ester side chain. This particular metabolitehas approximately 10% of the activity of the parentcompound. |
InChI:InChI=1/C20H24N2O6/c1-11(2)10-28-20(24)17-13(4)21-12(3)16(19(23)27-5)18(17)14-8-6-7-9-15(14)22(25)26/h6-9,11,18,21H,10H2,1-5H3
63675-72-9 Relevant articles
METHODS FOR TREATING CHRONIC FATIGUE SYNDROME AND MYALGIC ENCEPHALOMYELITIS
-
, (2021/03/13)
In one aspect the invention relates to a...
An efficient and recyclable 3D printed α-Al2O3 catalyst for the multicomponent assembly of bioactive heterocycles
Azuaje, Jhonny,Tubío, Carmen R.,Escalante, Luz,Gómez, Mónica,Guitián, Francisco,Coelho, Alberto,Caama?o, Olga,Gil, Alvaro,Sotelo, Eddy
, p. 203 - 210 (2016/12/09)
A catalytic methodology is reported that...
THERAPY FOR COMPLICATIONS OF DIABETES
-
, (2009/07/02)
A method for enhancing glycemic control ...
ANTIHYPERTENSIVE THERAPY
-
, (2009/09/08)
A new use of darusentan is provided in p...
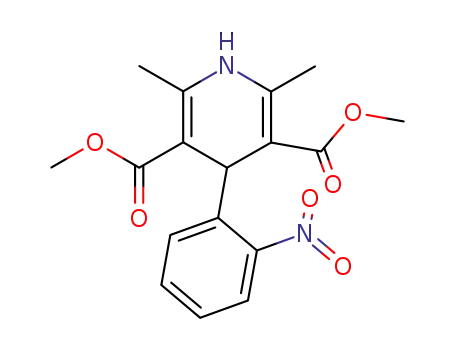
63675-72-9 Process route
-
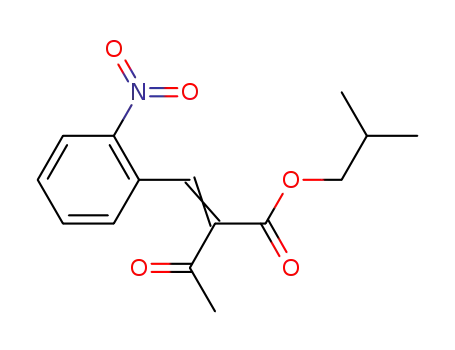
-
61312-59-2
2-(2-nitrobenzylidene)-acetoacetic acid-i-butyl ester

-
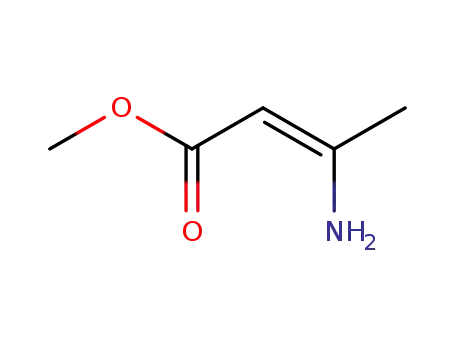
-
21731-17-9
methyl 3-aminocrotonate

-
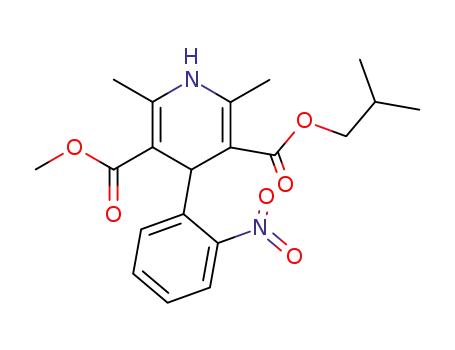
-
63675-72-9,103573-38-2,103573-36-0
nisoldipine
| Conditions | Yield |
|---|---|
|
With
dmap;
In
cyclohexane;
at 75 - 85 ℃;
for 26h;
Heating / reflux;
|
52.9% |
-
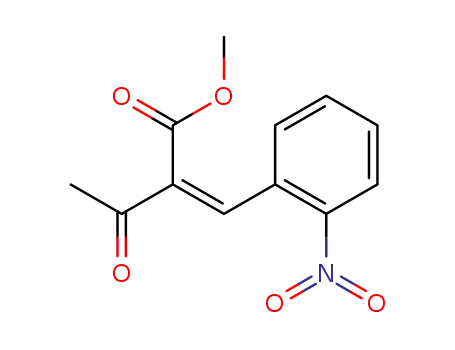
-
111304-31-5
2'-nitrobenzylideneacetoacetic acid methyl ester

-
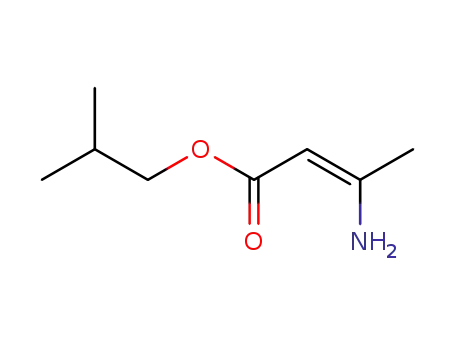
-
isobutyl 3-aminocrotonate

-

-
63675-72-9,103573-38-2,103573-36-0
nisoldipine
| Conditions | Yield |
|---|---|
|
In
1,2-dimethoxyethane; toluene;
at 80 - 105 ℃;
for 15 - 25h;
|
63675-72-9 Upstream products
-
61312-59-2

2-(2-nitrobenzylidene)-acetoacetic acid-i-butyl ester
-
21731-17-9

methyl 3-aminocrotonate
-
111304-31-5

2'-nitrobenzylideneacetoacetic acid methyl ester
-
21829-25-4
nifedipine
63675-72-9 Downstream products
-
103026-83-1
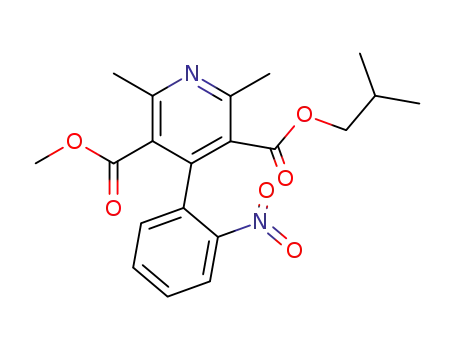
2,6-dimethyl-4-(2-nitrophenyl)-3,5-pyridinedicarboxylic acid methyl 2-methylpropyl ester
-
87375-91-5
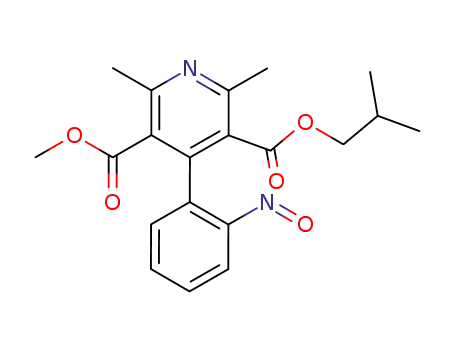
(+/-)-3-Isobutyl-5-methyl-1,4-dihydro-2,6-dimethyl-4-(2-nitrosophenyl)pyridine-3,5-dicarboxylate
-
103573-38-2
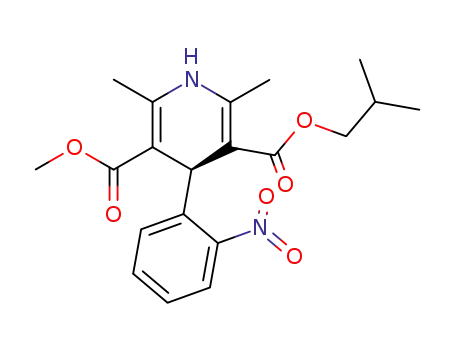
(+)-(S)-methyl 2-methylpropyl 1,4-dihydro-2,6-dimethyl-4-(2-nitrophenyl)pyridine-3,5-dicarboxylate
-
103573-38-2
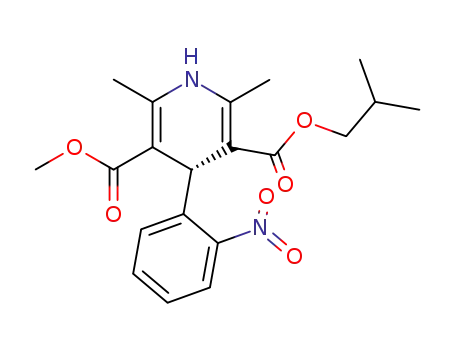
(-)-(R)-methyl 2-methylpropyl 1,4-dihydro-2,6-dimethyl-4-(2-nitrophenyl)pyridine-3,5-dicarboxylate
Relevant Products
-
Sodium carboxymethyl cellulose,CMC-Na
CAS:9004-32-4
-
L-HPC LH-11
CAS:9004-65-3
-
Nifedipine
CAS:21829-25-4