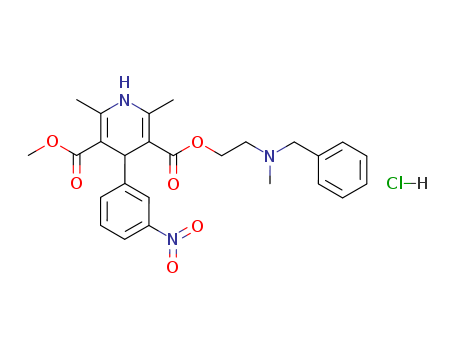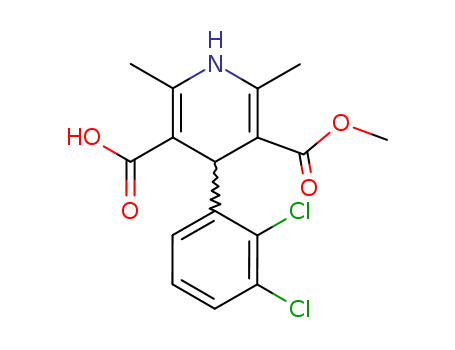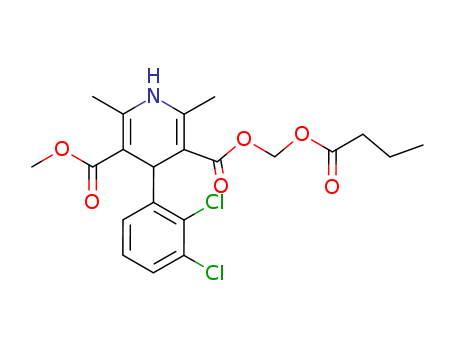
167221-71-8
- Product Name:Clevidipine butyrate
- Molecular Formula:C21H23Cl2NO6
- Purity:99%
- Molecular Weight:456.323
Product Details;
CasNo: 167221-71-8
Molecular Formula: C21H23Cl2NO6
Appearance: Powder
Factory Sells Best Quality Clevidipine butyrate 167221-71-8 with GMP standards
- Molecular Formula:C21H23Cl2NO6
- Molecular Weight:456.323
- Appearance/Colour:Powder
- Melting Point:128-130°C
- Boiling Point:539.7 °C at 760 mmHg
- PKA:2.45±0.70(Predicted)
- Flash Point:280.2 °C
- PSA:90.93000
- Density:1.289 g/cm3
- LogP:4.57400
Clevidipine butyrate(Cas 167221-71-8) Usage
|
Side effects |
Clevidipine is contraindicated in patients with severe aortic stenosis, defective lipid metabolism, and allergies to eggs, egg products, soybeans, or soy products. The chemical synthesis of clevidipine entails the esterification of 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic acid monomethyl ester with chloromethyl butyrate by the action of potassium bicarbonate in refluxing acetonitrile. |
|
Synthesis |
The chemical synthesis of clevidipine entails the esterification of 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic acid monomethyl ester with chloromethyl butyrate by the action of potassium bicarbonate in refluxing acetonitrile. The DHP-monoester starting material is obtained in two steps through condensation of methyl 2,3-dichlorobenzylidine acetoacetate and 2-cyanoethyl ester of 2-amino-2-propenoic acid to the DHP diester, followed by base catalyzed cleavage of the cyanoethyl group. Clevidipine is practically insoluble in water and is formulated in an oil-inwater emulsion. |
InChI:InChI=1/C21H23Cl2NO6/c1-5-7-15(25)29-10-30-21(27)17-12(3)24-11(2)16(20(26)28-4)18(17)13-8-6-9-14(22)19(13)23/h6,8-9,18,24H,5,7,10H2,1-4H3
167221-71-8 Relevant articles
Butyric acid clevidipine preparation method
-
Paragraph 0040; 0060-0061, (2017/01/17)
The invention discloses a preparation me...
Butyric acid clevidipine synthetic method
-
Paragraph 0052-0054, (2016/10/10)
The invention discloses a synthetic meth...
PROCESS FOR PREPARATION OF CLEVIDIPINE AND ITS INTERMEDIATE
-
Page/Page column 6, (2012/06/15)
The present invention relates to a proce...
PREPARATION OF INTERMEDIATES FOR THE SYNTHESIS OF DIHYDROPYRIDINE CALCIUM CHANNEL BLOCKERS
-
Page/Page column 7, (2011/11/06)
4- (2,3- dichlorophenyl) -1,4- dihydro- ...
167221-71-8 Process route
-
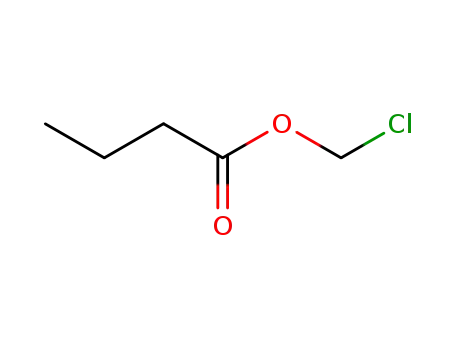
-
33657-49-7
chloromethyl n-butyrate

-
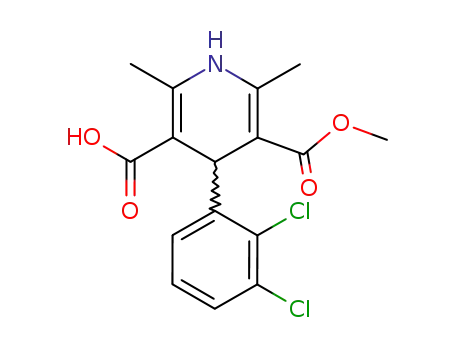
-
123853-39-4
(+)-4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-5-methoxycarbonyl-3-pyridinecarboxylic acid

-
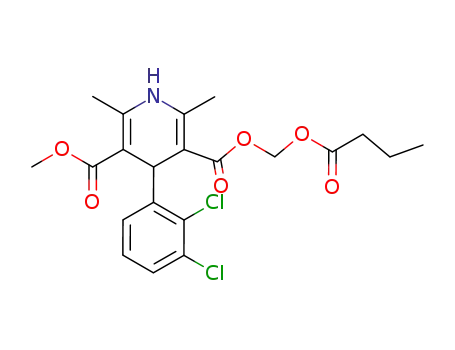
-
166432-28-6,167221-71-8
clevidipine
| Conditions | Yield |
|---|---|
|
With
potassium carbonate; lithium iodide;
In
N,N-dimethyl-formamide;
at 70 ℃;
for 4h;
Reagent/catalyst;
Temperature;
Solvent;
|
87.2% |
|
With
potassium carbonate;
In
1,4-dioxane; water;
for 4h;
Reflux;
|
66% |
|
With
sodium hydrogencarbonate;
In
N,N-dimethyl-formamide;
at 80 ℃;
for 4h;
|
65% |
|
(+)-4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-5-methoxycarbonyl-3-pyridinecarboxylic acid;
With
tetramethyl ammoniumhydroxide;
In
1,4-dioxane; water;
at 20 ℃;
for 0.75h;
chloromethyl n-butyrate;
In
1,4-dioxane; water;
for 5h;
Reflux;
|
|
|
With
sodium hydrogencarbonate;
In
N,N-dimethyl-formamide;
at 80 ℃;
for 4h;
Product distribution / selectivity;
Inert atmosphere;
|
-
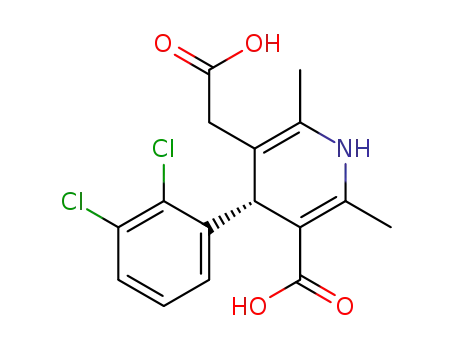
-
(4R)-1,4-dihydro-2,6-dimethyl-4-(2',3'-dichorophenyl)-5-carboxymethyl-3-pyridinecarboxylic acid

-

-
33657-49-7
chloromethyl n-butyrate

-

-
167356-39-0,166432-28-6,167221-71-8
(S)-Clevidipine
| Conditions | Yield |
|---|---|
|
With
sodium hydrogencarbonate;
In
dichloromethane; N,N-dimethyl-formamide;
|
70% |
167221-71-8 Upstream products
-
33657-49-7

chloromethyl n-butyrate
-
123853-39-4

(+)-4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-5-methoxycarbonyl-3-pyridinecarboxylic acid
-
1020718-27-7
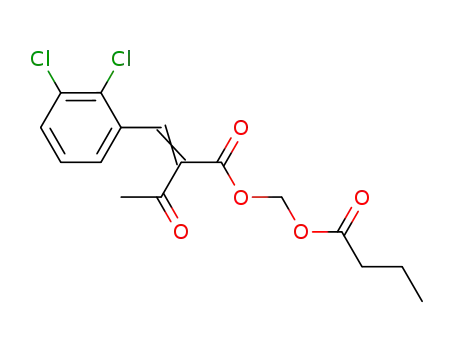
2-(2',3'-dichloro-benzylidene)-3-oxo-butyric acid butyryloxymethyl ester
-
14205-39-1
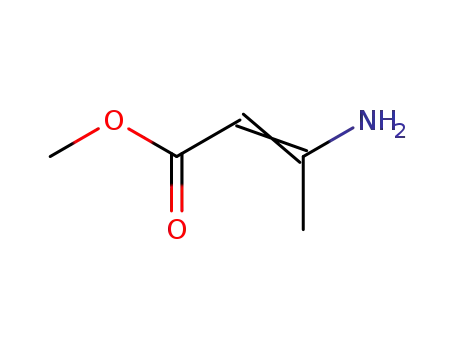
methyl 3-aminocrotonate
Relevant Products
-
Nicardipine hydrochloride
CAS:54527-84-3
-
(R)-3-Amino-Hexahydro-1H-Azepin
CAS:124932-43-0