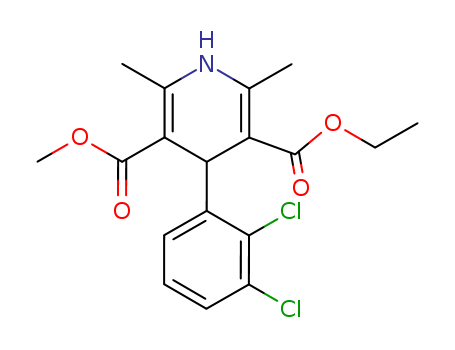
72509-76-3
- Product Name:Felodipine
- Molecular Formula:C18H19Cl2NO4
- Purity:99%
- Molecular Weight:384.259
Product Details;
CasNo: 72509-76-3
Molecular Formula: C18H19Cl2NO4
Appearance: White or light yellow crystalline powder
Reputable supplier selling Felodipine 72509-76-3 with stock
- Molecular Formula:C18H19Cl2NO4
- Molecular Weight:384.259
- Appearance/Colour:White or light yellow crystalline powder
- Vapor Pressure:4.62E-09mmHg at 25°C
- Melting Point:142-145 °C
- Refractive Index:1.549
- Boiling Point:471.5 °C at 760 mmHg
- PKA:2.73±0.70(Predicted)
- Flash Point:239 °C
- PSA:64.63000
- Density:1.277 g/cm3
- LogP:4.29310
Felodipine(Cas 72509-76-3) Usage
|
Manufacturing Process |
Preparation of 2,3-dichlorobenzylideneacetylacetic acid-methylester.2,3-Dichlorobenzaldehyde is reacted with methyl acetoacetate in a suitable solvent in the presence of a catalytic amount of acetic acid and piperidine. Water is azeotropically separated off during the reaction. The reaction mixture is extracted in order to remove the catalysts. The solvent is evaporated and methanol is added. The product is crystallized by cooling the solution, isolated by filtration and finally washed with methanol. |
|
Therapeutic Function |
Antihypertensive |
|
Biological Activity |
L-type Ca 2+ channel blocker that is selective over N-, R-, P/Q- and T-type channels. Displays high vascular selectivity; lowers arterial blood pressure without altering cardiac contractility. Antihypertensive. |
|
Definition |
ChEBI: The mixed (methyl, ethyl) diester of 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic acid. A calcium-channel blocker, it lowers blood pressure by reducing peripheral vascular resistance through a highly selective action on smooth m scle in arteriolar resistance vessels. It is used in the management of hypertension and angina pectoris. |
|
Brand name |
Plendil (AstraZeneca). |
|
General Description |
Felodipine, 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylic acid ethylmethyl ester (Plendil), is a second-generation dihydropyridinechannel blocker of the nifedipine type. It is more selectivefor vascular smooth muscle than for myocardial tissueand serves as an effective vasodilator. |
InChI:InChI=1/C18H19Cl2NO4/c1-5-25-18(23)14-10(3)21-9(2)13(17(22)24-4)15(14)11-7-6-8-12(19)16(11)20/h6-8,15,21H,5H2,1-4H3
72509-76-3 Relevant articles
An improved synthesis of high-purity felodipine
Yiu, Sai-Hay,Knaus, Edward E.
, p. 91 - 95 (1996)
-
METHODS FOR TREATING CHRONIC FATIGUE SYNDROME AND MYALGIC ENCEPHALOMYELITIS
-
, (2021/03/13)
In one aspect the invention relates to a...
Preparation method for felodipine
-
Paragraph 0014; 0040-0042; 0045-0048; 0051-0053, (2018/12/13)
The invention discloses a preparation me...
An efficient and recyclable 3D printed α-Al2O3 catalyst for the multicomponent assembly of bioactive heterocycles
Azuaje, Jhonny,Tubío, Carmen R.,Escalante, Luz,Gómez, Mónica,Guitián, Francisco,Coelho, Alberto,Caama?o, Olga,Gil, Alvaro,Sotelo, Eddy
, p. 203 - 210 (2016/12/09)
A catalytic methodology is reported that...
Metal-free-mediated oxidation aromatization of 1,4-dihydropyridines to pyridines using visible light and air
Wei, Xiaojing,Wang, Lin,Jia, Wenliang,Du, Shaofu,Wu, Lizhu,Liu, Qiang
supporting information, p. 1245 - 1250 (2015/02/05)
A metal-free and environmentally friendl...
72509-76-3 Process route
-
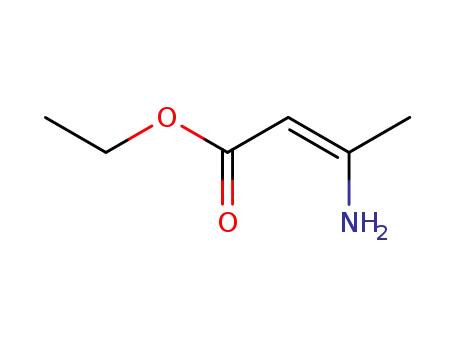
-
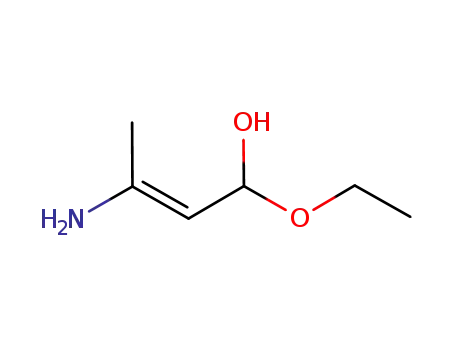
626-34-6
ethyl 3-aminobut-2-enoate

-
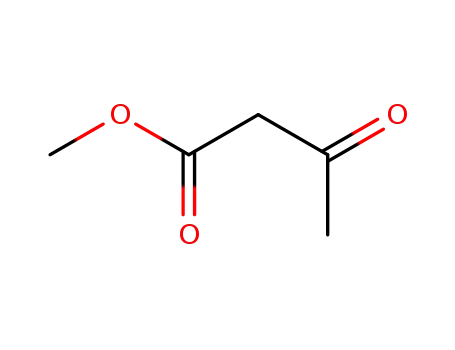
-
105-45-3
acetoacetic acid methyl ester

-
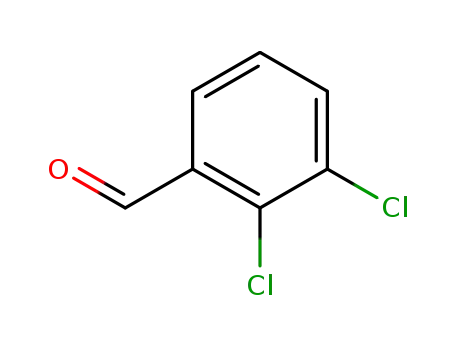
-
6334-18-5
2,3-dichlorobenzylaldehyde

-
-
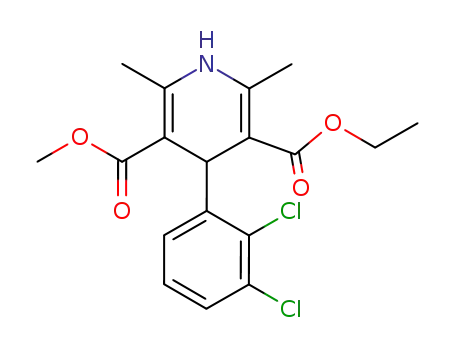
72509-76-3,86189-69-7
ethyl methyl 1,4-dihydro-2,6-dimethyl-4(2,3-dichlorophenyl)-3,5-pyridinedicarboxylate
| Conditions | Yield |
|---|---|
|
ethyl 3-aminobut-2-enoate; acetoacetic acid methyl ester; 2,3-dichlorobenzylaldehyde;
With
piperidine; pyridine;
In
neat (no solvent);
at 75 - 80 ℃;
for 9h;
With
ethanol;
for 1h;
Reagent/catalyst;
Solvent;
Reflux;
|
94.3% |
-
-
ethyl aminocrotonate

-

-
105-45-3
acetoacetic acid methyl ester

-

-
6334-18-5
2,3-dichlorobenzylaldehyde

-

-
72509-76-3,86189-69-7
ethyl methyl 1,4-dihydro-2,6-dimethyl-4(2,3-dichlorophenyl)-3,5-pyridinedicarboxylate

-
-
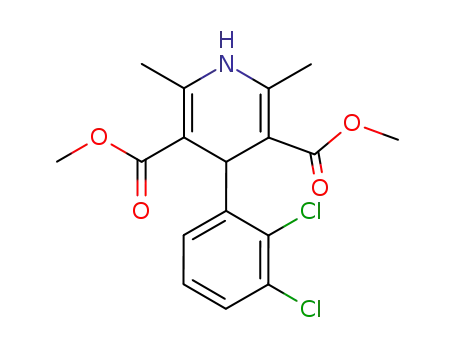
91189-59-2
4-(-2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylic acid methyl ester

-
-
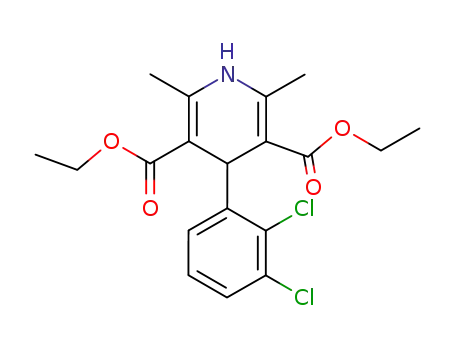
79925-38-5
4-(2,3-dichloro-phenyl)-2,6-dimethyl-1,4-dihydro-pyridine-3,5-dicarboxylic acid diethyl ester
| Conditions | Yield |
|---|---|
|
acetoacetic acid methyl ester; 2,3-dichlorobenzylaldehyde;
piperidine; 2-Picolinic acid;
In
isopropyl alcohol;
at 40 - 45 ℃;
for 6h;
ethyl aminocrotonate;
In
isopropyl alcohol;
for 4h;
Heating / reflux;
|
66% |
72509-76-3 Upstream products
-
68064-64-2
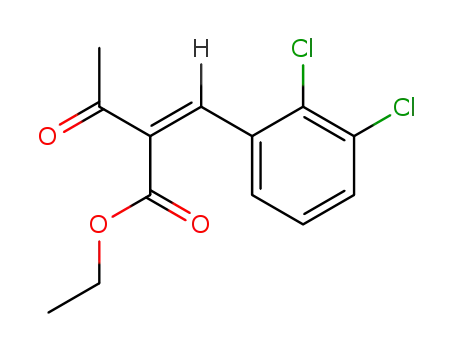
2-<(2,3-dichlorophenyl)methylene>-3-oxobutanoic acid ethyl ester
-
21731-17-9
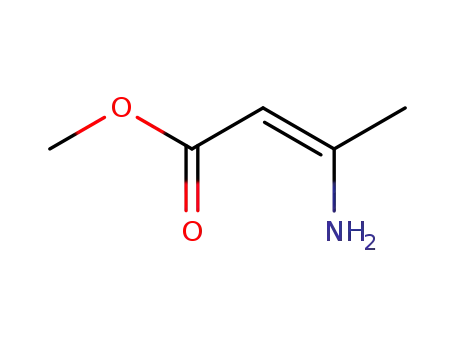
methyl 3-aminocrotonate
-
74-88-4
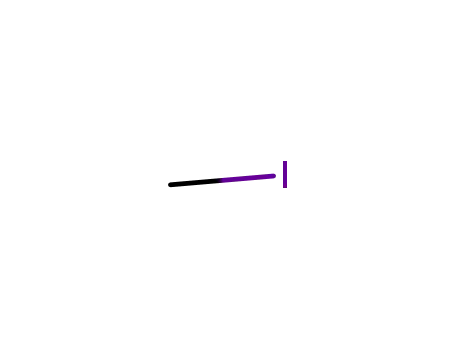
methyl iodide
-
81777-30-2
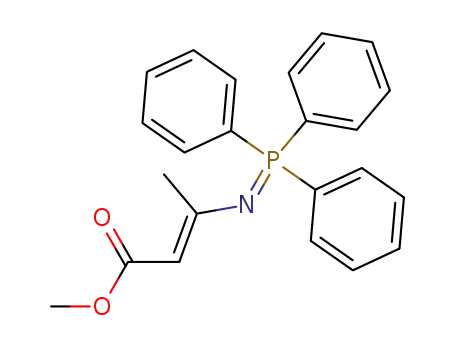
1,1,1-Triphenyl-3-methyl-4-(methoxycarbonyl)-2-aza-1λ5-phosphabuta-1,3-diene
72509-76-3 Downstream products
-
119945-59-4
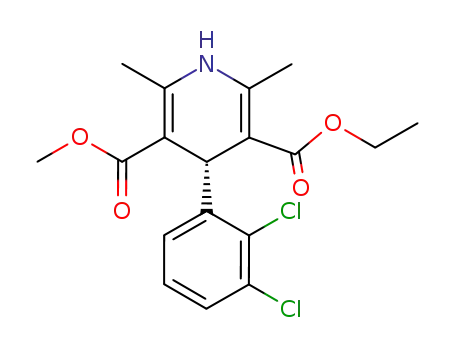
(R)-Felodipine
-
105618-03-9
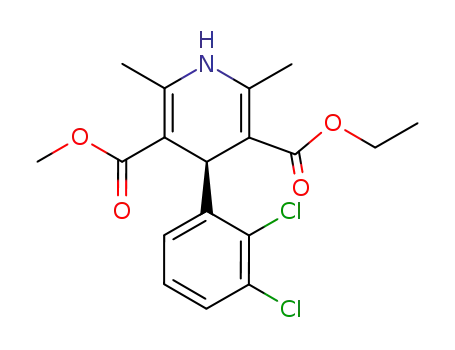
(S)-Felodipine
-
96382-71-7
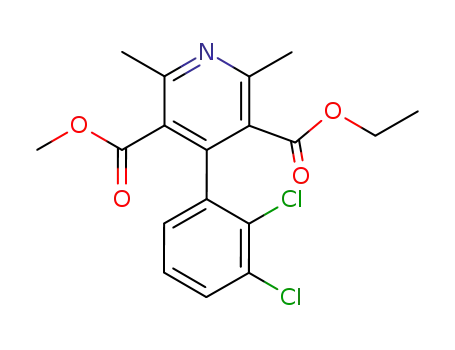
Dehydrofelodipine
Relevant Products
-
Nicardipine hydrochloride
CAS:54527-84-3
-
Hydroxypropyl starch (HPS),Creamell
CAS:9049-76-7
-
(R)-3-Amino-Hexahydro-1H-Azepin
CAS:124932-43-0