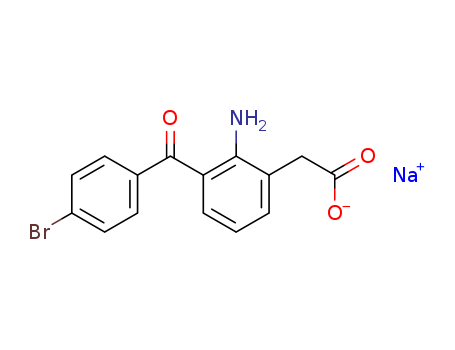
91714-93-1
- Product Name:Bromfenac sodium
- Molecular Formula:C15H11BrNNaO3
- Purity:99%
- Molecular Weight:356.151
Product Details;
CasNo: 91714-93-1
Molecular Formula: C15H11BrNNaO3
Manufacturer supply top purity Bromfenac sodium 91714-93-1 with GMP standards
- Molecular Formula:C15H11BrNNaO3
- Molecular Weight:356.151
- Vapor Pressure:1.77E-13mmHg at 25°C
- Melting Point:285 °C
- Boiling Point:562.2 °C at 760 mmHg
- Flash Point:293.8 °C
- PSA:83.22000
- LogP:2.13590
Bromfenac sodium(Cas 91714-93-1) Usage
|
Manufacturing Process |
Reaction of (2-aminophenyl)-(4-bromophenyl)-methanone with methylsulfanylacetic acid ethyl ester and tert-butyl hypochlorite gives a corresponding sulfonium salt. This salt was transformed to initially to the betaine. Electrocyclic rearrangement of that transient intermediate leads, after rearomatization, to the homoanthranilic acid. Internal ester-amine interchange leads then to 4-bromophenyl-(3-(methylthio)indolin-7-yl)methanone. The thiomethyl group is then removed with Raney nickel to give 4-bromophenyl- (indolin-7-yl)methanone. Saponification of this intermediate affords the (2- amino-3-(4-bromobenzoyl)-phenyl)-acetic acid (Bromfenac). In practice it is usually used as sodium salt. |
|
Therapeutic Function |
Analgesicá Antiinflammatory |
|
Biochem/physiol Actions |
Bromfenac exhibits antipyretic and prostaglandin synthetase inhibiting properties. It has therapeutic properties against the reduction of ocular pain and inflammation in postoperative cataract patients. Bromfenac acts as an effective agent against allergic conjunctivitis. It has the potential to treat acute muscle pain, osteoarthritis, and rheumatoid arthritis. |
|
Definition |
ChEBI: The sodium salt of bromfenac. Note that 'bromfenac sodium' commonly refers to the sesquihydrate (120638-55-3); this is the anhydrous form. |
|
Brand name |
Duract |
InChI:InChI=1/C15H12BrNO3.Na.H2O/c16-11-6-4-9(5-7-11)15(20)12-3-1-2-10(14(12)17)8-13(18)19;;/h1-7H,8,17H2,(H,18,19);;1H2/q;+1;/p-1
91714-93-1 Relevant articles
Novel synthesis method of bromfenac sodium
-
Paragraph 0073; 0096-0112; 0135-0151; 0174-0189, (2021/11/27)
The novel synthesis method is characteri...
Refining method and preparation method of bromfenac sodium sesquihydrate
-
Paragraph 0098-0100, (2021/01/29)
The invention relates to a refining meth...
Simple preparation method of bromfenac sodium
-
Paragraph 0077-0085, (2020/06/09)
The invention relates to a simple prepar...
Preparation method of bromfenac sodium
-
, (2020/03/29)
The invention provides a preparation met...
91714-93-1 Process route
-
![7-(4-bromophenyl)-3-carboxymethylbenzo[c]isoxazole](/upload/2024/12/b753c292-893f-4494-af0e-a8acb215aa1b.png)
-
7-(4-bromophenyl)-3-carboxymethylbenzo[c]isoxazole

-
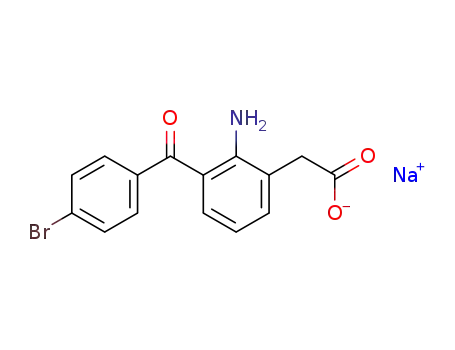
-
91714-93-1
bromfenac sodium
| Conditions | Yield |
|---|---|
|
7-(4-bromophenyl)-3-carboxymethylbenzo[c]isoxazole;
With
5%-palladium/activated carbon; hydrogen;
In
methanol; water;
at 25 - 35 ℃;
for 4h;
under 2250.23 - 3750.38 Torr;
Autoclave;
Cooling with ether-dry ice;
With
sodium hydroxide;
In
water;
at 20 - 25 ℃;
for 2h;
Reagent/catalyst;
Temperature;
Pressure;
|
96.3% |
-
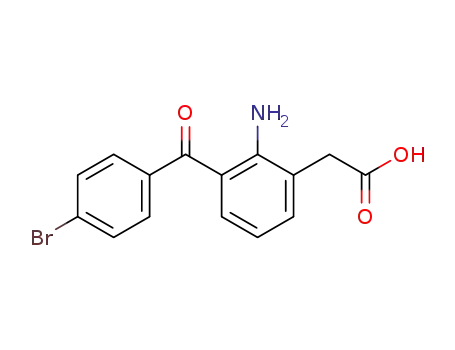
-
91714-94-2
bromfenac

-

-
91714-93-1
bromfenac sodium
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
ethanol; toluene;
at 30 - 35 ℃;
pH=11 - 14;
Temperature;
|
95% |
|
With
sodium hydroxide;
In
water;
at 5 - 10 ℃;
for 1h;
Autoclave;
|
90.35% |
91714-93-1 Upstream products
-
91713-91-6
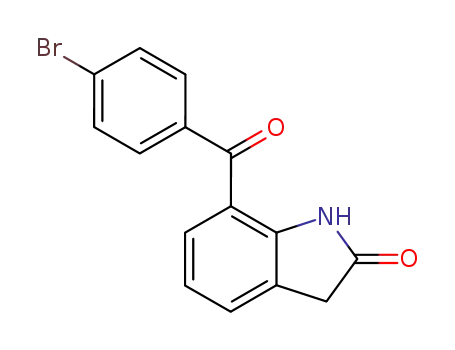
7-(4-bromobenzoyl)-1,3-dihydro-2H-indol-2-one
-
91714-50-0
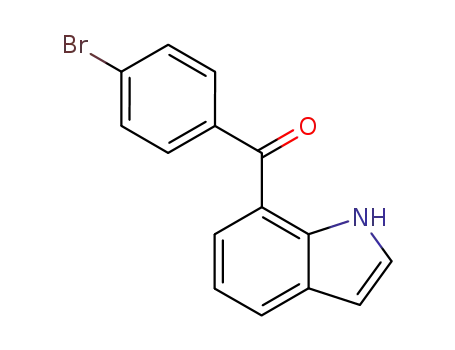
7-(4-bromobenzoyl)-1-hydro-indole
-
91714-94-2

bromfenac
-
1140-17-6
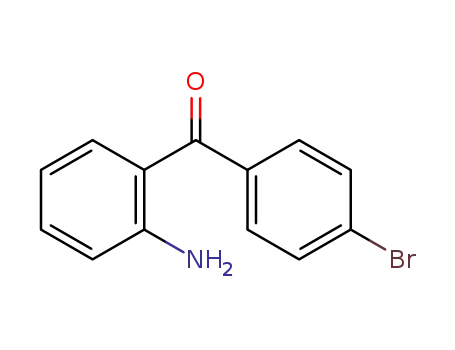
2-amino-4'-bromobenzophenone
91714-93-1 Downstream products
-
91713-91-6

7-(4-bromobenzoyl)-1,3-dihydro-2H-indol-2-one
Relevant Products
-
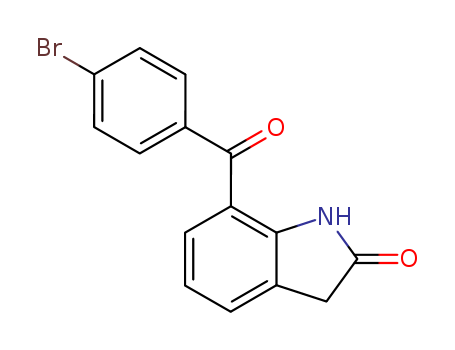
7-(4-Bromobenzoyl)-1,3- dihydro-2H-indol-2-one
CAS:91713-91-6
-
Sodium carboxyl methylstarch
CAS:9063-38-1
-
Loteprednol etabonate
CAS:82034-46-6