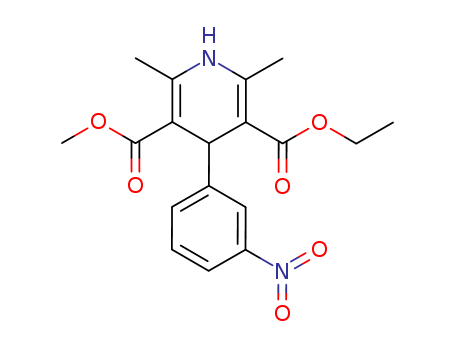
39562-70-4
- Product Name:Nitrendipine
- Molecular Formula:C18H20N2O6
- Purity:99%
- Molecular Weight:360.367
Product Details;
CasNo: 39562-70-4
Molecular Formula: C18H20N2O6
Appearance: Crystalline solid
Factory Sells Best Quality Nitrendipine 39562-70-4 with ISO standards
- Molecular Formula:C18H20N2O6
- Molecular Weight:360.367
- Appearance/Colour:Crystalline solid
- Vapor Pressure:8.08E-09mmHg at 25°C
- Melting Point:158 °C
- Refractive Index:1.579
- Boiling Point:488.9 °C at 760 mmHg
- PKA:2.79±0.70(Predicted)
- Flash Point:249.5 °C
- PSA:110.45000
- Density:1.247 g/cm3
- LogP:3.41770
Nitrendipine(Cas 39562-70-4) Usage
|
Biochem/physiol Actions |
Ca2+ channel blocker; anti-hypertensive. |
|
Definition |
ChEBI: A dihydropyridine that is 1,4-dihydropyridine substituted by methyl groups at positions 2 and 6, a 3-nitrophenyl group at position 4, a ethoxycarbonyl group at position 3 and a methoxycarbonyl group at position 5. It is a calcium-channel blocker used in t e treatment of hypertension. |
|
Brand name |
Baypress (Bayer);BAYOTENSIN. |
|
General Description |
Nitrendipine, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinecarboxylic acid methyl ethylester (Baypress), is a second-generation dihydropyridinechannel blocker of the nifedipine type. It is more selectivefor vascular smooth muscle than for myocardial tissue andserves as an effective vasodilator. The drug is used in thetreatment of mild-to-moderate essential hypertension. |
InChI:InChI=1/C18H20N2O6/c1-5-26-18(22)15-11(3)19-10(2)14(17(21)25-4)16(15)12-7-6-8-13(9-12)20(23)24/h6-9,15-16H,5H2,1-4H3/t15?,16-/m1/s1
39562-70-4 Relevant articles
Facile synthesis of 1,4-dihydropyridine monocarboxylic acids and unsymmetric dicarboxylates via quaternary ammonium salts of 2-aminoethyl 1,4-dihydropyridine-3,5-dicarboxylates
Kinugawa, Masahiko,Ogasa, Takehiro
, p. 3321 - 3331 (1997)
Useful 1,4-dihydropyridine unsymmetric d...
METHODS FOR TREATING CHRONIC FATIGUE SYNDROME AND MYALGIC ENCEPHALOMYELITIS
-
, (2021/03/13)
In one aspect the invention relates to a...
Preparation method of nitrendipine
-
Paragraph 0051-0071, (2020/05/14)
The invention belongs to the technical f...
Preparation method of nitrendipine
-
Paragraph 0042-0058, (2019/05/22)
The invention belongs to the field of dr...
Based on the three-step synthesis process of preparation of the nitrendipine (by machine translation)
-
Paragraph 0020; 0025; 0031-0032; 0035-0036; 0041-0042, (2019/02/04)
The invention discloses a method based o...
39562-70-4 Process route
-
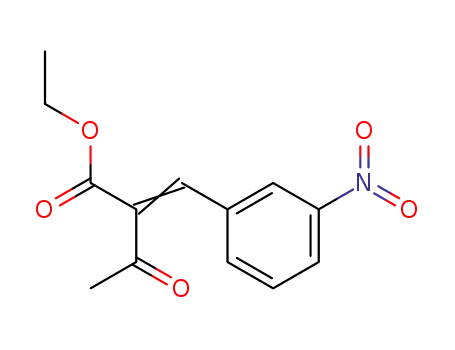
-
39562-16-8
ethyl 2-(3-nitrophenylmethylene)-3-oxobutanoate

-
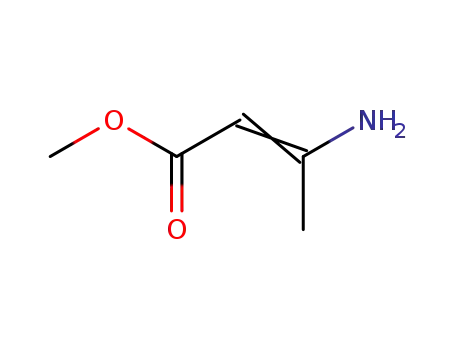
-
14205-39-1
methyl 3-aminocrotonate

-
-
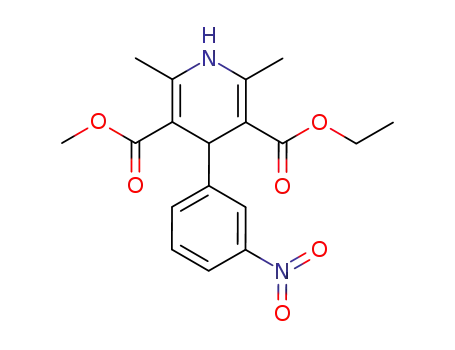
39562-70-4
1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridine-dicarboxylic acid ethyl methyl ester
| Conditions | Yield |
|---|---|
|
ethyl 2-(3-nitrophenylmethylene)-3-oxobutanoate; methyl 3-aminocrotonate;
In
ethanol;
at 70 - 75 ℃;
for 1h;
With
hydrogenchloride;
In
ethanol; water;
at 70 - 75 ℃;
|
80% |
|
In
ethanol;
for 8h;
Heating;
|
79% |
|
With
acetic acid; N-ethyl-N,N-diisopropylamine;
In
ethanol;
Reflux;
|
-

-
21731-17-9
methyl 3-aminocrotonate

-
![ethyl 2-[(3-Nitrophenyl)methylene]-3-oxobutanoate](/upload/2024/12/fbffdcd0-d42e-4967-ba65-a2b542542786.png)
-
39562-16-8
ethyl 2-[(3-Nitrophenyl)methylene]-3-oxobutanoate

-

-
39562-70-4
1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridine-dicarboxylic acid ethyl methyl ester
| Conditions | Yield |
|---|---|
|
methyl 3-aminocrotonate; ethyl 2-[(3-Nitrophenyl)methylene]-3-oxobutanoate;
In
ethanol;
at 75 - 80 ℃;
for 1h;
With
acetic anhydride;
In
ethanol;
at 75 - 80 ℃;
for 1h;
Solvent;
|
86% |
39562-70-4 Upstream products
-
119128-13-1
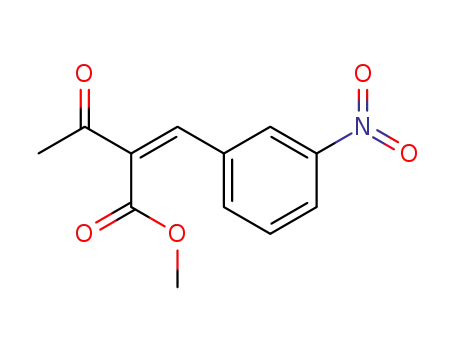
methyl (Z)-2-(3-nitrobenzylidene)-3-oxobutanoate
-
141-97-9
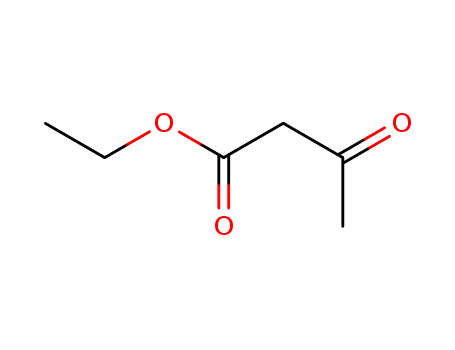
ethyl acetoacetate
-
99-61-6
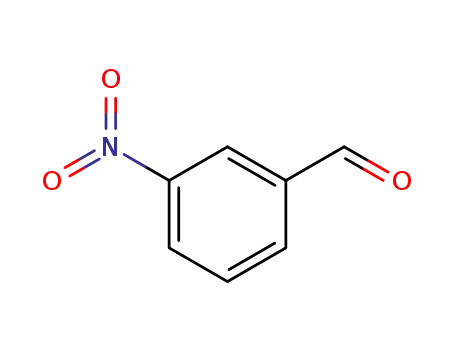
3-nitro-benzaldehyde
-
14205-39-1
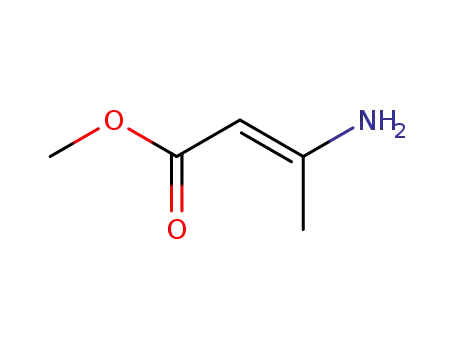
methyl (E)-3-aminocrotonate
39562-70-4 Downstream products
-
123754-04-1
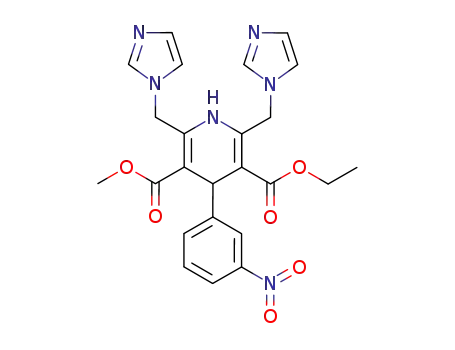
1,4-dihydro-2,6-bis(1H-imidazol-1-ylmethyl)-4-(3-nitrophenyl)pyridine-3,5-dicarboxylic acid ethyl methyl diester
-
64603-72-1
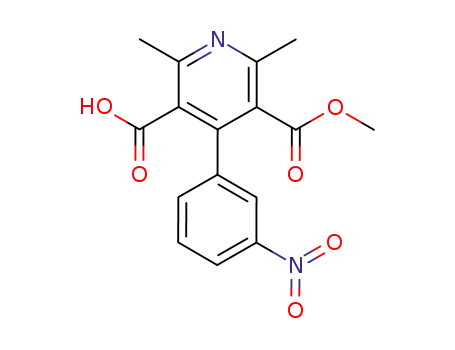
SL 5721
-
74936-72-4
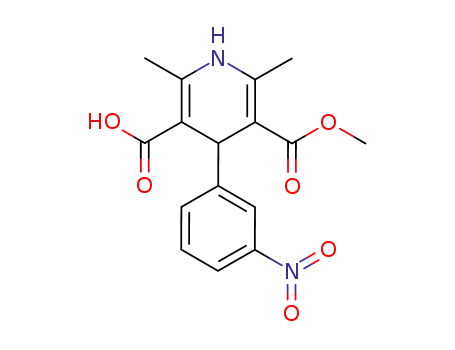
5-methoxycarbonyl-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carboxylic acid
-
64603-74-3
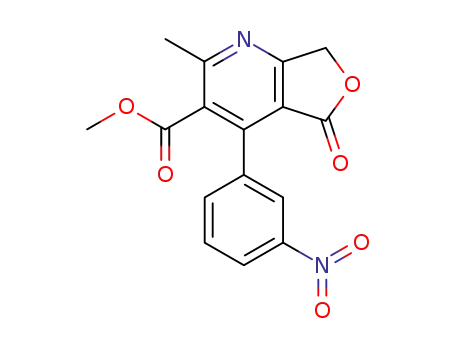
methyl 2-methyl-4-(3-nitrophenyl)-5-oxo-5,7-dihydro-furo<3,4-b>pyridine-3-carboxylate
Relevant Products
-
Sodium carboxymethyl cellulose,CMC-Na
CAS:9004-32-4
-
HPMC E4M
CAS:9004-65-3
-
HPMC K50M
CAS:9004-65-3